Product Description
REAGEN Endotoxins are complex lipopolysaccharides (LPS) , which are biologically active structural components of the outer cell membrane of gram-negative bacteria with a molecular weight of around 10 kDa and their general structure consists of three parts: a lipid component containing fatty acids and disaccharide phosphates (Lipid A), O-specific polysaccharide side chains (Oligo S) and a core polysaccharide chain (O Poly S). Removal of endotoxin in biological product is one of the key
criteria. Monitor and quantitatively measure the endotoxin level is required. The mainstream LAL method is time-consuming and lack a broad linear response range. REAGEN endotoxin fluorescent kit is a quick and accurate assay to quantitate the endotoxin level in a biological product.
Procedure Overview
REAGEN Endotoxin Test Kit adopts the re-engineered rFc protein (the protease domain is replaced by human trypsin I). When endotoxins bind to rFc N terminal domain, rFc’s confirmation changes, the protease domain PRSS1 is exposed and activated. The colorimetric substrate pNA peptide added in the reaction mixture is therefore cleaved, and free pNA has a peak absorbance at 405 nm. 405 nm absorbance value is proportional to the endotoxin level in the samples.
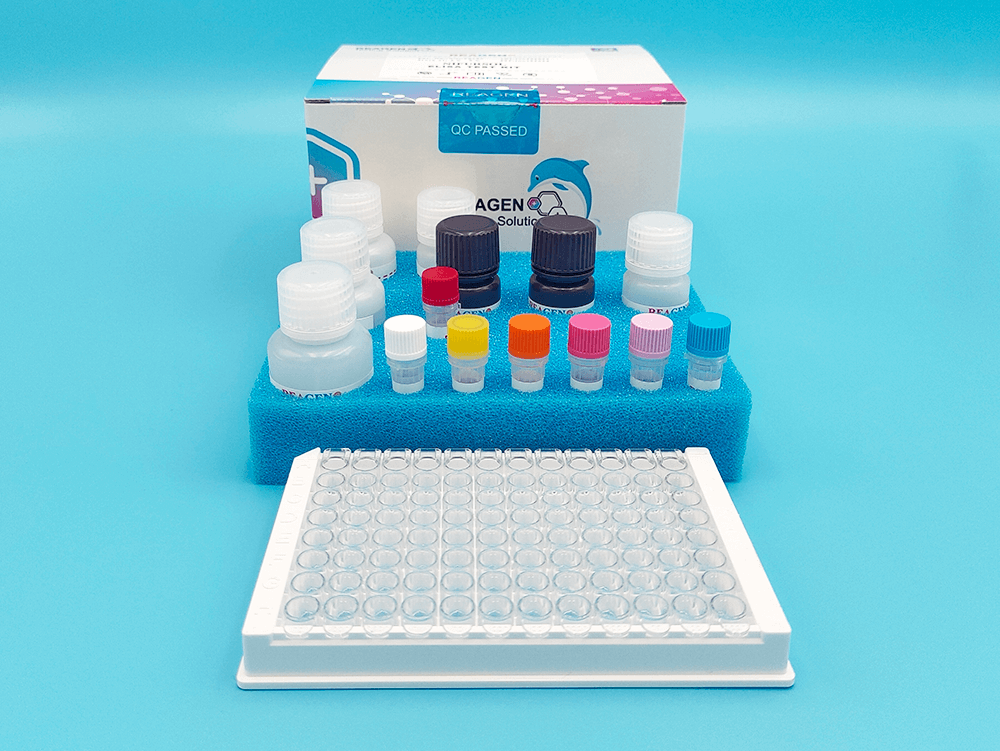
Kit Contents, Storage and Shelf Life
Store the kit at -20°C . The shelf life is 12 months when the kit is properly stored.
| Kit Contents | Amount | Storage |
| Lyophilized rFc Enzyme | 1 |
-20°C |
| Fluorescent Substrate | 1 | |
| Bacterial LPS Standards:
STD A 0.05 EU/mL STD B 0.15 EU/mL STD C 0.45 EU/mL STD D 1.35 EU/mL STD E 4.05 EU/mL STD F 12.15 EU/mL STD G 36.45 EU/mL |
200 µL 200 µL 200 µL 200 µL 200 µL 200 µL 200 µL |
|
| Stop Buffer | 10 mL |
Required Materials Not Provided With the Kit
- ELISA microplate reader (with 405 nm filter).
- ELISA microplate.
- Pipette and pipette tips
- Software for data analysis (or Microsoft Excel, or Open Office calc etc.)
- Timer
Warnings and Precautions
- Do not use the kit past the expiration date.
- Do not intermix reagents from different kits or lots except for components with the same part No’s within their expiration dates.
- Try to maintain a laboratory temperature of 20°–25°C (68°–77°F). Avoid running assays under or near air vents, as this may cause excessive cooling, heating and/or evaporation. Also, do not run assays in direct sunlight, as this may cause excessive heat and evaporation. Cold bench tops should be avoided by placing several layers of paper towel or some other insulation material under the assay plates during incubation.
- Make sure you are using only distilled or deionized water since water quality is very important.
- When pipetting samples or reagents into an empty microtiter plate, place the pipette tips in the lower corner of the well, making contact with the plastic.
- Incubations of assay plates should be timed as precisely as possible. Be consistent when adding standards to the assay plate. Add your standards first and then your samples.










Reviews
There are no reviews yet.